ANTIBIOTIC RESISTANT BACTERIA
What is antibiotic resistance?
Antibiotic resistance is an ability acquired by germs like bacteria and fungi, to defeat the drugs designed to kill them. So, the germs are not killed and continue to grow. Most importantly, in antibiotic resistance, the body does not become resistant to antibiotics; it is that bacteria have become resistant against the antibiotics designed to kill them.
As a result of this, infections caused by antibiotic resistant germs are difficult or sometimes impossible to treat. In most cases, antibiotic resistant infections require extended hospital stays, additional follow-up doctor visits, and costly and toxic alternatives. The danger is that antibiotic resistance has the ability to affect people at any age and as well for any professionals including healthcare, veterinary, and agriculture industries. This has made antibiotic resistance one of the world’s most urgent public health problems.
According to the records, each year in the USA, at least 2.8 million people are infected with antibiotic resistant microbes, and among them more than 35,000 people die. Completely avoiding the risk of antibiotic resistant infections is impossible. But people with chronic illnesses are at greater risk than others. When antibiotics lose their effectiveness, then the ability to treat infections is lost and it becomes hard to control public health threats. Many medical treatments including joint replacements, organ transplants, cancer therapy and treatments for chronic diseases like diabetes, asthma, and rheumatoid arthritis are dependent on the ability of antibiotics to fight against infections.
Factors Causing Antimicrobial Resistance
- The widespread use of antimicrobial agents without strictly adhering to the scientific principles guiding their use.
- Laws to restrict sale of non-prescribed antimicrobial agents do not exist.
- Doctors prescribe antimicrobial agents upon patient insistence.
- Common misconception among people that antibiotics are needed during the viral infection of a common cold.
- The tendency to miss doses or use antimicrobial agents over a long term with gap periods.
- Use of antibiotics as livestock feed.
- The release of large volumes of antimicrobial agents into water bodies by pharmaceutical companies without proper waste water treatment .
Microbial resistance to drugs is acquired through…

Four main biochemical mechanisms are responsible for bacterial resistance to a given antibiotic. They are,
- The impermeability of the bacterial membrane or cell wall to the antibiotic
- The reduction of the antibiotic’s affinity for its bacterial target(s), by quantitative or qualitative modification of the latter
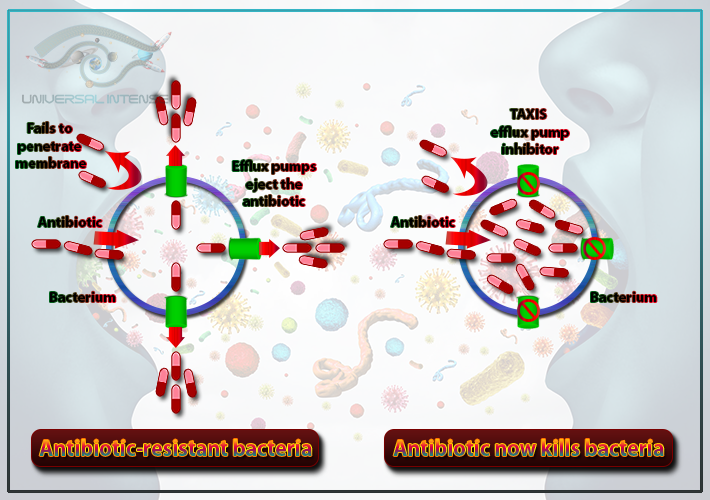
- The efflux of the antibiotic out of the bacteria after penetration
- The inactivation of the antibiotic by bacterial enzymes
Bacteria restrict access by changing the entryways or limiting the number of entryways. Some bacteria including Staphylococcus aureus produce a thickened cell wall which makes it difficult for the antibiotic to enter the cell. Gram-negative bacteria use their outer membrane to selectively keep antibiotic drugs from entering.
Multiple components in the bacterial cell act as targets of antimicrobial drugs and many of them may be modified by the bacteria to enable resistance to those drugs. This changes the antibiotic’s target in such a way that the drug can no longer fit and do its job. Gram positive bacteria show resistance to the β-lactam drugs via alterations in the structure and/or number of PBPs (penicillin-binding proteins). Gram negative bacteria acquire resistance against these drugs via genes that result in changes in the peptidoglycan precursors’ structure. Resistance to drugs that target the ribosomal subunits may occur via ribosomal mutation and ribosomal subunit methylation. Bacteria show resistance to drugs that target nucleic acid synthesis, via modifications in DNA gyrase or topoisomerase IV enzymes. For the drugs which inhibit metabolic pathways, the resistance is acquired via mutations in enzymes involved in the folate biosynthesis pathway.
Bacteria use pumps in their cell walls to get rid of antibiotic drugs that enter the cell. These pumps are known as multi-drug [MDR] efflux pumps since many of these pumps will transport several different important antibiotic drugs. Available carbon sources influence the resistance capability of many of these pumps.
Bacteria inactivate the antibiotic by two main ways. They are,
- By actual degradation of the drug
- By transfer of a chemical group to the drug
The β-lactamases are an example for drug hydrolyzing enzymes. Drug inactivation is done by transfer of a chemical group to the drug and most commonly used transfer groups are acetyl, phosphoryl, and adenyl.
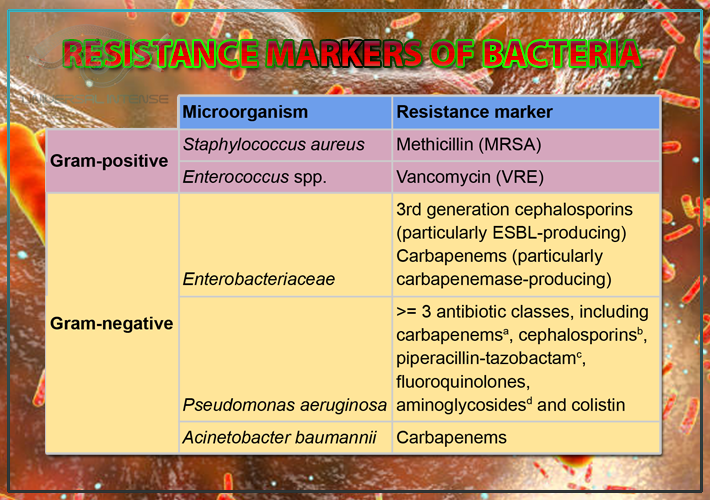
Resistance markers of bacteria
Both gram-positive and gram-negative bacteria show resistance against antibiotics including methicillin, vancomycin, cephalosporins, carbapenems, piperacillin-tazobactam, fluoroquinolones, aminoglycosides and colistin.
What are antibiotic resistant bacteria?
Important examples for bacteria which have the capability of causing serious disease due to the resistance to most commonly available antibiotics are mentioned below.
- Carbapenem resistant Acinetobacter baumannii
- Vancomycin resistant Enterococcus
- Pseudomonas aeruginosa antibiotic resistance
- Methicillin-resistant Staphylococcus aureus
- Carbapenem resistant Klebsiella pneumoniae (CRKP)
- Multi-drug-resistant Mycobacterium tuberculosis (MDR-TB)
- Multidrug-resistant Escherichia coli
Carbapenem resistant Acinetobacter baumannii
- Causes penumonia, urinary tract and bloodstream infections
- Infections are usually hospital acquired
- New mechanism by which bacteria resist colistin antibiotic revealed
These infections tend to occur in patients in intensive care units because of their frequent contamination of healthcare facility surfaces and medical equipment. So infection control measures should be taken including rigorous cleaning and disinfection. A decrease can be seen in the overall rates of carbapenem-resistant Acinetobacter cases. Mobile genetic elements including DNA are carried by carbapenem-resistant Acinetobacter and they are easily shareable between other germ producing bacteria. This amplifies the problem of resistance because carbapenem-resistant Acinetobacter that can produce carbapenemases appear to be increasing. It is an enzyme which destroys these important drugs by making carbapenem antibiotics ineffective and spreads resistance rapidly. This reduces patient treatment options further and threatens to reverse decreases of carbapenem-resistant Acinetobacter cases. There are Acinetobacter species which are resistant to nearly all antibiotics. So a few new drugs are in the developmental step now.
Vancomycin resistant Enterococcus

- Bacterial type resistance
- Causes serious infections for patients in healthcare settings, including bloodstream, surgical site, and urinary tract infections
- Estimated cases in hospitalized patients in 2017 is about 54,500
- Estimated deaths in 2017 is about 5,400
Among all healthcare-associated enterococcal infections, about 30% are resistant to vancomycin. This causes the reduction of treatment options. Nearly all Vancomycin resistant Enterococcus infections can be seen in patients with healthcare exposures. There are several risk factors for Vancomycin resistant Enterococcus infection. Some of them are staying in long-term care hospitals or intensive care units (ICUs), undergoing organ transplant, or receiving treatment for certain cancer types. Vancomycin resistant Enterococcus has increased resistance to additional antibiotics and this has raised concern that the remaining drugs to treat Vancomycin resistant Enterococcus may become less effective.
Patients who undergo complex or prolonged health care or patients with weakened immune systems are at high risk for Vancomycin resistant Enterococcus infections. One type of Vancomycin resistant Enterococcus can be seen in solid organ transplant units. It is Enterococcus faecium (E. faecium) and known as the most common cause of central line-associated bloodstream infections (CLABSIs). Nearly 70% of these E. faecium are resistant to vancomycin and as a result of this healthcare providers are reliant on other antibiotics. The number of Vancomycin resistant Enterococcus infections can be further reduced by maintaining and improving infection prevention and control interventions including hand hygiene and surface disinfection.
Pseudomonas aeruginosa antibiotic resistance
- Bacterial type resistance
- Usually occur in people with weakened immune systems
- Particularly dangerous for patients with chronic lung diseases
- Estimated cases in hospitalized patients in 2017 is about 32,600
- Estimated deaths in 2017 is about 2,700
Infections of Pseudomonas aeruginosa usually occur in hospitalized people or people with weakened immune systems. Patients with chronic lung diseases can be affected by this more than other people. Certain types of multidrug-resistant (MDR) P. aeruginosa are resistant to nearly all antibiotics, including carbapenems. Among 2% to 3% of them carry a mobile genetic element which makes a carbapenemase enzyme. As a result of this enzyme, carbapenem antibiotics become ineffective. Since mobile genetic elements are easily shareable between bacteria, the resistance is spread rapidly which results in destroying these important drugs.
According to the records of 2018, CDC’s Antibiotic Resistance Laboratory Network has identified an outbreak of carbapenem-resistant P. aeruginosa which forms an unusual type of resistance. The outbreak included more than 20 people across several states. According to the reviews of health departments, those patients had undergone at least one surgery in a hospital. So, most of the patients had surgical site infections and some required prolonged hospitalization in the USA. Immediate action has been taken by CDC and partners to implement the Containment Strategy by notifying the USA health departments, Canadian and Mexican public health authorities, and the World Health Organization. Hundreds of patients were notified of their risk for possible exposure to carbapenem-resistant P. aeruginosa, helping to protect these patients and contain spread.
Methicillin-resistant Staphylococcus aureus
- Bacterial type resistance
- Also known as “Resistant staph” (short for Staphylococcus)
- A common bacteria that spread in healthcare facilities and the community
- Cause difficult-to-treat staph infections because of resistance to some antibiotics
- Estimated cases in hospitalized patients in 2017 is about 323,700
- Estimated deaths in 2017 is about 10,600
Several treatments are available for this, but still Methicillin-resistant Staphylococcus aureus has become resistant to many first-line antibiotics. Overall amount of Methicillin-resistant Staphylococcus aureus infections are decreasing. But progress of Methicillin-resistant Staphylococcus aureus bloodstream infections prevention in healthcare has slowed. People who inject drugs are 16 times more likely to develop a serious (invasive) Methicillin-resistant Staphylococcus aureus infection than those who do not. Methicillin-resistant Staphylococcus aureus infections can be prevented through effective infection control interventions. Between 2005 and 2017, Veterans Affairs (VA) medical centers rates of Methicillin-resistant Staphylococcus aureus have decreased by 55%. The reason for this success was implementation of Centers for Disease Control and prevention (CDC) recommended interventions at 153 VA hospitals across the USA. They have taken steps to prevent the spreading of Methicillin-resistant Staphylococcus aureus and device- and procedure-associated infections. Some of those steps are screening and tracking all patients for Methicillin-resistant Staphylococcus aureus on admission, using Contact Precautions including gloves, gowns for people with Methicillin-resistant Staphylococcus aureus, and highlighting the importance on hand hygiene. Other than that changes in institutional culture, has also made preventing Methicillin-resistant Staphylococcus aureus infections the responsibility of any VA employee taking care of patients. And also, employee adherence to infection prevention practices was tracked. As a result of that many hospitals outside of the VA system have also successfully reduced Methicillin-resistant Staphylococcus aureus rates. This has been done by assessing facility data and implementing CDC-recommended prevention strategies.
Carbapenem resistant Klebsiella pneumoniae (CRKP)
Carbapenem-resistant Klebsiella pneumoniae (CRKP) is considered as one of the most serious global threats since carbapenems are known as the agents of last resort in the treatment of life-threatening infections caused by drug-resistant Enterobacteriaceae. Carbapenem-resistant Enterobacteriaceae (CRE) were listed as one of the most urgent antibiotic resistance threats by the CDC and WHO. Carbapenem resistance can be seen mainly among Enterobacteriaceae, especially Klebsiella pneumoniae and was first found a decade ago while subsequently emerging in several countries. K. pneumoniae carbapenemase (KPC) is designated by one particular group of transmissible plasmid-encoded carbapenemase enzymes and this resistance is rapidly spreading worldwide. Resistance to “last-line” drugs, such as colistin, is emerging in CRKP strains, and some isolates are resistant to all antibiotics tested. KPC is an Ambler class A carbapenemase and mediates resistance to extended-spectrum cephalosporins in addition to carbapenems. Carbapenem-resistant K. pneumoniae (CRKP) can cause several types of healthcare-associated infections such as pneumonia, bloodstream, wound, surgical site and urinary tract infections and meningitis. Most importantly, this resistivity is associated with high mortality rates. The nosocomial transmission of CRKP can be reduced by following effective strategies, including hand hygiene, contact precautions, minimizing invasive device use, promoting antimicrobial stewardship, screening, active surveillance testing and chlorhexidine bathing. Moreover, the rapid notification of clinical infection whenever CRKP are identified from clinical specimens allows the timely implementation of control measures.
Multi-drug-resistant Mycobacterium tuberculosis (MDR-TB)
Mycobacterium tuberculosis spreads through air and is breathed into lungs. Regular TB kills around 2 million people a year, mostly in poor countries lacking access to medicine. Multi-drug-resistant Mycobacterium tuberculosis (MDR-TB) is known as a dangerous variant of the tuberculosis bacteria. This strain thwarts the two most powerful of first line drugs. The reason for Multidrug-resistant tuberculosis (MDR-TB) is, bacteria that do not respond to, at least, isoniazid and rifampicin which are known as the two most powerful anti-TB drugs. The drug resistance in Multidrug-resistant Mycobacterium tuberculosis is caused by incorrect prescription, poor quality drugs, erratic supply of drugs and patient non-adherence. According to the latest anti-TB drug resistance surveillance data, 3.5% of new and 18% of previously treated TB cases in the world are estimated to be multidrug-resistant. About 8.5% of MDR-TB cases had extensively drug-resistant TB (XDR-TB). It is resistant to the two most powerful anti-TB drugs of the four first line (standard) drugs against TB and to three or more of six classes of second-line drugs which are more expensive, less effective drugs used when first-line fails. Extensively drug-resistant TB (XDR-TB) was first described in 2006 and more than 5000 cases reported worldwide. In 2015, only 55% of the MDR/RR-TB patients who started treatment globally were successfully treated, whereas 15% of patients died and 8% of patients failed in treatment. The treatment success in XDR-TB patients was only 34% in that year.
Multidrug-resistant Escherichia coli
Escherichia coli can frequently cause life-threatening bloodstream infections and other common infections including urinary tract infections. Antibiotic resistance rates in E. coli are increasing rapidly. This can be seen especially towards fluoroquinolones (enrofloxacin) and third- and fourth-generation cephalosporins (ceftiofur and cefquinome). Most of these antibiotic-resistant strains of E. coli (resistant to fluoroquinolones and extended-spectrum β-lactams) are acquired in the community rather than in healthcare settings. Although many countries, especially some developing countries, use more of these critically important antibiotics than the United States does. Recently, the US Food and Drug Administration has limited the use of antibiotics including ceftiofur. The antibiotic-resistant strains that people in the community carry will affect the types and numbers of antibiotic-resistant bacteria seen in healthcare facilities. The antibiotic resistance can be seen in bacteria recovered from patients in healthcare settings. Poor infection control and antibiotic practices of the hospital may often cause this. Almost all antibiotic-resistant strains gain the ability to proliferate and spread in healthcare settings due to the exposure of patients to antibiotics which are being used to treat pneumonia or to provide surgical prophylaxis.
Antibiotic | Percentage of the resistivity |
penicillin | 100% |
erythromycin | 100% |
nalidixic acid | 98% |
cephalexin | 94% |
amoxicillin | 86% |
ampicillin | 84% |
ciprofloxacin | 74% |
tetracycline | 64% |
cefixime | 54% |
gentamicin | 36% |
Penicillin resistance
Penicillin is given to patients with bacterial infections including pneumonia, strep throat, meningitis, syphilis and gonorrhea referred to the National Library of Medicine. Other than that it can be used to prevent dental infections.
The first reports of strains highly resistant to penicillin began to appear in the 1980s. Some bacterial strains have developed resistance to penicillin. Some of them are,
- Staphylococcus aureus
- Neisseria gonorrhoeae
- Enterococcus strains
- Streptococcus pneumoniae
- Streptococcus viridans
1. Staphylococcus aureus

Penicillin-resistant Staphylococcus aureus (golden staph) appeared in the clinic with the widespread use of penicillin in the 1950s. They can produce penicillinase, which has the ability to hydrolyze the penicillin β-lactam ring, leading to resistance to penicillin. They acquire this resistance through plasmid mediated transduction, transformation, and insertion of drug-resistant genes. This leads to the excessive β-lactamase production resulting in bacterial resistance. Mainly the plasmids or drug-resistant gene transmission is mediated by plasmids and these result in the genome expanding. Further, resistance genes can be transferred between Staphylococcus aureus and other bacteria. For example, penicillin-resistant Staphylococcus aureus can obtain drug-resistant plasmids from penicillin-resistant Enterococcus while expanding and enhancing its resistance further.
2. Neisseria gonorrhoeae
Neisseria gonorrhoeae (the cause of gonorrhoea) has developed high resistance towards penicillin due to the overuse of penicillin. It has been acquired through two main mechanisms:
- Chromosomally mediated resistance (CMRNG)
- Penicillinase-mediated resistance (PPNG)
Chromosomally mediated resistance occurred through stepwise changes over many years. This mechanism falls under the category of second general mechanism for beta-lactam resistance. PBPs, generally known as transpeptidases, are the targets for beta-lactams and they are involved in peptidoglycan synthesis, a major component of the bacterial cell wall. The amino acid strands of peptidoglycan are cross linked by PBS during synthesis and beta-lactams bind the PBPs while inhibiting the cross linking of peptidoglycan. As a result of this the bacterium cell wall is compromised and often results in cell death. Penicillinase-mediated resistance in N. gonorrhoeae is mediated by the plasmid borne TEM-1 type beta-lactamase and it falls under the category of third general mechanism for beta-lactam resistance. Among over 200 beta-lactamases described, some are antibiotic specific and TEM-1 is a penicillinase which is specific for penicillins. Penicillinase will bind to the beta-lactam ring which is known as a structural characteristic for beta-lactams and follow by hydrolyzing the ring. As a result of this the antibiotic becomes inactive. Since the plasmids containing TEM-1 could be passed among bacteria via conjugation, the spread of the penicillinase resistance was much faster compared with chromosomally mediated resistance mechanisms.
3. Enterococcus strains
As mentioned before Enterococci strains usually acquire antibiotic resistance through exchange of resistance encoding genes which are carried on conjugative transposons, pheromone responsive plasmids, and other broad host range plasmids. Issues in the treatment of enterococcal infections were noticed in the early 1950s when it was observed that enterococcal endocarditis was not cured nearly as often as streptococcal endocarditis using penicillin. The reason for this poor response is that penicillin is not much bactericidal for enterococci as it is for most viridans-group streptococci. This phenomenon is known as tolerance and it is characteristic for enterococcal strains. The tolerance can be rapidly elicited using intermittent pulsed penicillin exposure in those that do not display tolerance initially.
4. Streptococcus pneumoniae
Streptococcus pneumoniae have resistance towards β-lactams through modifying their penicillin binding proteins (PBPs), that make up the active site of transpeptidase enzymes. Normally β-lactams react with PBPs and form a relatively stable ring-opened product. But when there are mutations in the genetic coding for proteins making up the transpeptidase enzyme, it would result in multiple PBPs with a low binding affinity towards β-lactams. As a result of this, the β-lactam ‘key’ no longer fits into the transpeptidase active site ‘lock’. Final outcome of this is that penicillins do not show an effective binding to their target PBPs. So, the transpeptidase enzymes continuously function and bind with their original substrate rather than the penicillin.
5. Streptococcus viridans
Streptococcus viridans cause infections in both adults and children. It is transmitted through coughs and sneezes and minor infections caused by Streptococcus viridans can be treated relatively easily with antibiotics. Penicillin-resistant Streptococcus viridans have been isolated in increasing numbers from various samples including from the mouth, blood, and wounds. For the past 5 decades, Streptococcus viridans isolated from patients with endocarditis have remained sensitive to penicillin. According to the studies, it is suggested that penicillin-resistant viridans streptococcal endocarditis should be treated in the same way as enterococcal endocarditis. Use of an aminoglycoside with the associated risk of renal or eighth nerve damage is also included here. There is little data to suggest that penicillin-resistant Streptococcus viridans are analogous to enterococci in their response to therapy. So, inclusion of an aminoglycoside may not be required in all cases. On the other hand, use of a penicillin alone for penicillin-resistant Streptococcus viridans is controversial.
How to avoid antimicrobial resistance
- implement infection prevention and control practices
- improve antibiotic use
- including ensuring access
- implement data and tracking systems to track resistance
- guide prevention strategies
- report results at the local and global level
- improve lab capacity to identify resistant bacteria